The PreciseGreen dsDNA quantification kit is used for the quantification of double-stranded DNA if its concentration cannot be determined by measuring absorbance at 260 nm. PreciseGreen selectively binds to double-stranded DNA, so nucleotides, single-stranded DNA, RNA, proteins and other impurities do not impede the measurements.
The linear measurement range for DNA concentration with this kit is 1 pg/μL to 5 ng/μL. The dye bound to double-stranded DNA exhibits an excitation maximum at 503 nm and a fluorescence emission maximum at 525 nm. Any type of fluorometer can be used for the assay read-out.
Kit components
| Kit component | Count | |||
|---|---|---|---|---|
|
1102-20 20 assays |
1102-200 200 assays |
B1102 200 assays |
||
| AA650, dsDNA quantitative standard, 100 ng/uL in TE buffer, 100 uL | 1 | — | — | |
| 42010, PreciseGreen® dsDNA Quantification Reagent, 200×, 1 mL | — | 1 | — | |
| 12010, PreciseGreen® dsDNA Quantification Reagent, 200×, 100 uL | 1 | — | 10 | |
| N2150, TE buffer, 20x, 25 mL | 1 | 1 | 1 | |
| BA650, dsDNA quantitative standard, 100 ng/uL in TE buffer, 1 mL | — | 1 | 1 | |
Store at temperature below 4 °С. Do not freeze! Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate.
Shelf life 12 months.
The amount of dye solution supplied with the kit is sufficient to analyze 200 experimental data points with an assay volume of 2 mL each (i.e. the minimum assay volume required for measurements in a standard fluorometric cuvette with a volume of 3.5 mL). The number of measurements may differ depending on the assay volume if other types of equipment are used for fluorescence detection. Recommended assay volumes for commonly used fluorometric equipment are provided in the table below.
Recommended volumes for dsDNA quantification with PreciseGreen dye:
| Type of equipment | Assay volume (Vassay) | Volume of PreciseGreen dye working solution | Volume of DNA sample | |
|---|---|---|---|---|
| Cuvette fluorometer | Standard fluorometric cuvette (3.5 mL) | 2 mL | 1 mL | 1 mL |
| Other fluorometric cuvettes | About 75 % of cuvette volume | 37.5 % of cuvette volume | 37.5 % of cuvette volume | |
| Plate fluorometer | 96-well plate*, per well | 0.2 mL | 0.1 mL | 0.1 mL |
| 24-well plate, per well | 1 mL | 0.5 mL | 0.5 mL | |
| Other plates | About 75 % of well volume | 37.5 % of well volume | 37.5 % of well volume | |
| Micro-fluorospectrometer* | 0.1 mL | 0.05 mL | 0.05 mL | |
* To maintain accuracy and precision of your measurements, we recommend to avoid pipetting volumes smaller than 2 µL.
Protocol
! We recommend to prepare 10–25 % extra volume of the 1× TE buffer solution and dye working solution to account for possible pipetting errors.
1. Preparation of 1× TE buffer
Prepare a sufficient amount of 1× TE buffer taking into account the assay volume and the number of samples (plus 5 dilutions of the dsDNA quantitative standard, see item 3). To prepare 1× TE buffer, dilute the 20× TE concentrate 20-fold with deionized water (see the table above to determine the recommended assay volumes for the fluorometric equipment used).
Calculate the buffer volume (V1× buffer) with the following formula:
V1× buffer = Vassay × (Nsamples + 5),
where Vassay — assay volume of a sample or standard, Nsamples — number of samples, and 5 — number of standards (including a blank sample).
2. Preparation of the PreciseGreen dye working solution
Thaw the PreciseGreen dye stock solution, then mix thoroughly. Prepare a sufficient amount of the PreciseGreen dye working solution taking into account the number of samples. The volume of the PreciseGreen dye working solution for each experimental data point should be equal to 50 % of the assay volume. To prepare the PreciseGreen dye working solution, dilute the PreciseGreen stock solution 200-fold with 1× TE buffer.
! The dye working solution should to be used within 3 hours after preparation.
Calculate the volume of the PreciseGreen dye working solution (VPreciseGreen) with the following formula:
VPreciseGreen = 1/2 × Vassay × (Nsamples + 5),
where Vassay — assay volume of a sample or standard, Nsamples — number of samples, and 5 — number of standards (including blank sample).
! Use only plastic containers to prepare the dye working solution, as PreciseGreen can adsorb to glass surfaces, which results in decreasing of the dye concentration in samples and biases in the measurement results.
3. Preparation of DNA standards
Prepare a DNA stock solution with a concentration of 2 ng/µL in 1× TE buffer by mixing 30 µL of the dsDNA quantitative standard from the kit with 1.47 mL of 1× TE buffer. From this stock solution, prepare a dilution series of standard DNA solutions with the following concentrations: 2 ng/µL, 200 pg/µL, 20 pg/µL, 2 pg/µL (see the table below).
! The proposed dilution scheme for the DNA stock solution can be used for the preparation of a dilution series with DNA concentrations of 0 to 2 ng/μL. 1.5 mL of the DNA stock solution prepared according to this scheme is sufficient to prepare a dilution series for a 3.5 mL cuvette (assay volume 2 mL). For smaller assay volumes, the volume of the DNA stock solution can be reduced accordingly.
| Volume of 1× ТЕ buffer, µL | Volume of DNA stock solution 2 ng/µL, µL | Concentration of DNA standard solution | Final concentration of DNA standard in assay volume |
|---|---|---|---|
| 0 | 1000 | 2 ng/µL | 1 ng/µL |
| 900 | 100 | 200 pg/µL | 100 pg/µL |
| 990 | 10 | 20 pg/µL | 10 pg/µL |
| 999 | 1 | 2 pg/µL | 1 pg/µL |
| 1000 | 0 | 0 pg/µL | 0 pg/µL |
Mix each DNA standard solution with an equal volume of the dye working solution (for the assay volume refer to the table above).
! In the case of non-linear progression of the calibration curve near the limits of the instrument’s dynamic range, adjust the concentrations of the standard solutions to match the fluorometer’s capabilities.
4. Preparation of samples
Dilute each DNA sample with 1× TE buffer to a volume that equals 50 % of the assay volume. Add an equal volume of the PreciseGreen dye working solution and mix.
! The final DNA concentration after dilution with 1× TE buffer and addition of the PreciseGreen dye working solution should be in the range of 1 pg/μL to 5 ng/μL.
5. Incubate all prepared standard solutions and experimental DNA samples for 5 minutes at room temperature.
6. Fluorescence measurements
Measure the fluorescence emission intensity of all standard solutions and DNA samples with suitable filter settings. The dye bound to double-stranded DNA exhibits maximum absorption at 503 nm and maximum fluorescence emission at 525 nm.
7. Calculation of dsDNA concentration
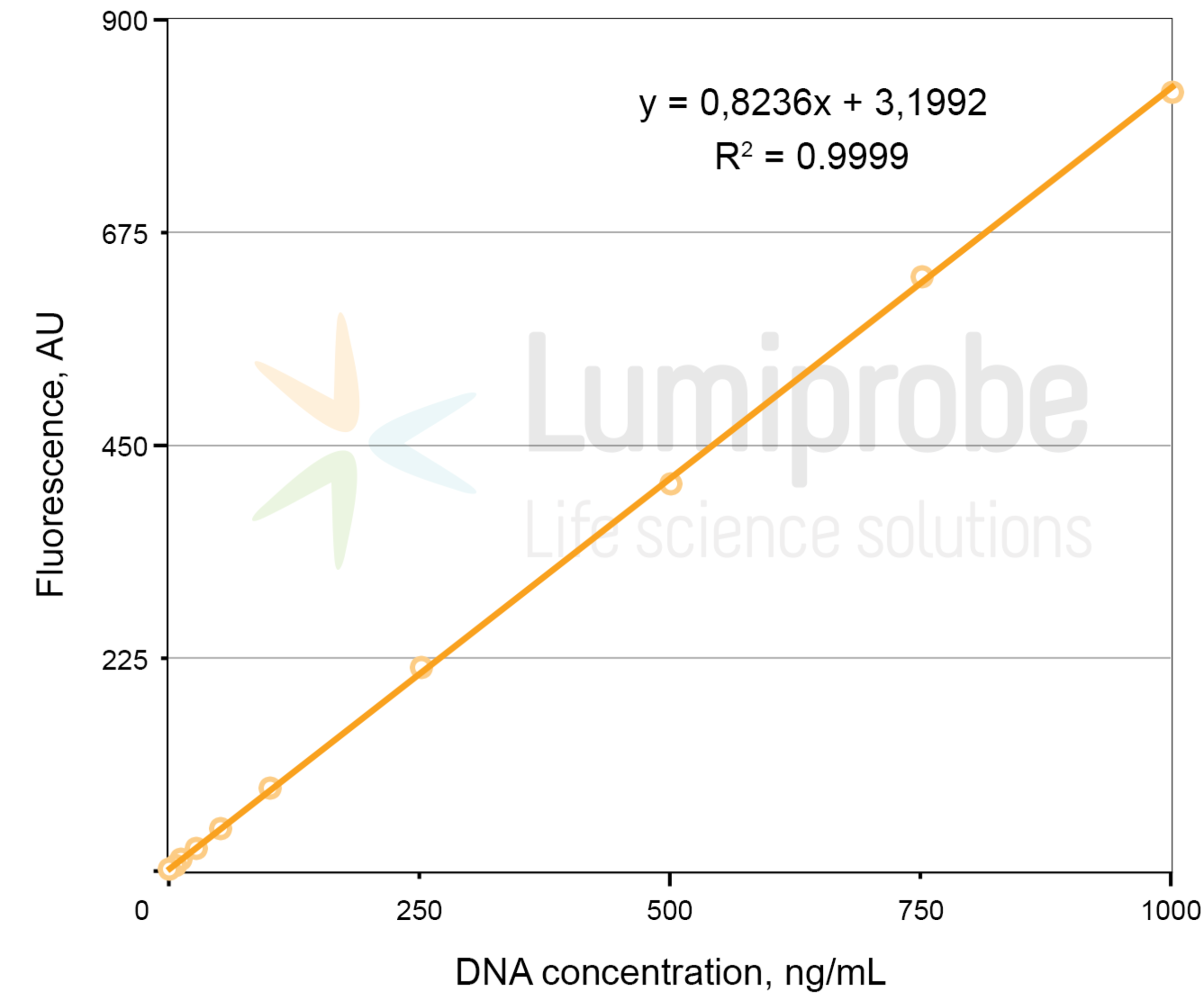
Create a calibration curve using the fluorescence values of the standard solutions. Fit a linear function to the data points to determine the function parameters A and B. The linear dependence between fluorescence (FL) and dsDNA concentration (C) is as follows:
FL= A × C + B, where FL — fluorescence intensity in arbitrary units, C — dsDNA concentration, A and B — parameters of the linear function.
DNA concentration in the diluted sample:
Csample = (FLsample − B)/A , where FLsample — fluorescence intensity of the diluted sample, A and B — parameters of the linear function.
DNA concentration in the initial sample:
Сinit = Vassay × Csample/Vinit , where Vassay — assay volume, Vinit — the volume of the initial sample used for dilution with 1× TE buffer.
The following online calculators can be used for the described calculations: dsDNA quantification calculator and Solubilization and dilution calculator .
Linear regression example: fluorescence versus DNA concentration
Related kits
PreciseGreen® dsDNA Quantification Kit
A kit for the quantification of dsDNA using PreciseGreen® fluorescent dye.| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 1102-20 |
20 assays
|
– | 1 days | |
| 1102-200
/ 1 mL dye
|
200 assays
|
$450
|
1 days | |
| B1102
/ 10 x 100 uL dye
|
200 assays
|
$500
|
1 days |







 $
$ 

