BDP® 630/650 amine
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 154C0 | 1 mg | $125 | in stock | |
| 254C0 | 5 mg | $260 | in stock | |
| 454C0 | 25 mg |
$510
|
in stock | |
| 554C0 | 50 mg |
$895
|
in stock | |
| 654C0 | 100 mg |
$1490
|
in stock |

BDP 630/650 is a far red emitting, borondipyrromethene based fluorophore. The dye is tuned to match the standard Cy5 channel, and can be used as an alternative to Cyanine5 and sulfo-Cyanine5. Compared to cyanines, BDP 630/650 possesses a longer fluorescence lifetime which is important for fluorescence anisotropy measurements.
BDP 630/650 has a brightness similar to cyanines, and an exceptional photostability.
This amine derivative is useful for the reaction with electrophiles, and for enzymatic transamination labeling.
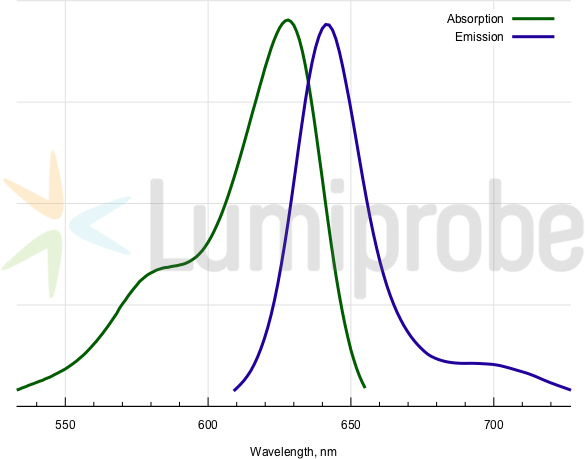
Absorption and emission spectra of BDP 630/650
Customers also purchased with this product
BDP® 558/568 DBCO
BDP 558/568 DBCO is a bright and photostable dye with emission in the yellow spectrum range. It contains a cyclooctyne substituting group (i.e. DBCO), which can react with various azides in Click Chemistry reactions.Cyanine3 NHS ester
Amine-reactive Cyanine3 dye NHS ester for the labeling of amino-groups.General properties
| Appearance: | dark violet solid |
| Molecular weight: | 584.92 |
| Molecular formula: | C29H32N4BClF2O2S |
| Solubility: | moderately soluble in water (81 mM = 47.2 mg/mL), well soluble in DMF, DMSO, alcohols |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 628 |
| ε, L⋅mol−1⋅cm−1: | 97000 |
| Emission maximum, nm: | 642 |
| Fluorescence quantum yield: | 0.91 |
| CF260: | 0.029 |
| CF280: | 0.035 |
Product citations
- Aitova, A.; Scherbina, S.; Berezhnoy, A.; Slotvitsky, M.; Tsvelaya, V.; Sergeeva, T.; Turchaninova, E.; Rybkina, E.; Bakumenko, S.; Sidorov, I.; Popov, M.A.; Dontsov, V.; Agafonov, E.G.; Efimov, A.E.; Agapov, I.; Zybin, D.; Shumakov, D.; Agladze, K. Novel Molecular Vehicle-Based Approach for Cardiac Cell Transplantation Leads to Rapid Electromechanical Graft–Host Coupling. International Journal of Molecular Sciences, 2023, 24(12), 10406. doi: 10.3390/ijms241210406
- Zhang, Y.; Zhu, X.; Chen, X.; Chen, Q.; Zhou, W.; Guo, Q.; Lu, Y.; Li, C.; Zhang, Y.; Liang, D.; Sun, T.; Wei, X.; Jiang, C. Activated Platelets-Targeting Micelles with Controlled Drug Release for Effective Treatment of Primary and Metastatic Triple Negative Breast Cancer. Advanced Functional Materials, 2019, 29(13), 1806620. doi: 10.1002/adfm.201806620
- Zhang, Y.; Guo, Z.; Cao, Z.; Zhou, W.; Zhang, Y.; Chen, Q.; Lu, Y.; Chen, X.; Guo, Q.; Li, C.; Liang, D.; Sun, T.; Jiang, C. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials, 2018, 183, 243–257. doi: 10.1016/j.biomaterials.2018.06.002











 $
$ 
