sulfo-Cyanine3 carboxylic acid
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 11390 | 1 mg | $110 | in stock | |
| 21390 | 5 mg | $190 | in stock | |
| 41390 | 25 mg | $390 | in stock | |
| 51390 | 50 mg |
$695
|
in stock | |
| 61390 | 100 mg |
$1190
|
in stock |

Water soluble sulfo-Cyanine3 dye, free unactivated monofunctional carboxylic acid. This reagent can be used as a reference fluorophore for Cy3® detection channel, as a control in experiments with other sulfo-Cyanine3 labeled products. Carboxylic acid can be also activated with carbodiimides.
Absorbance and emission spectra are identical with Cy3® fluorophore.
Sulfo-Cyanine3 absorbance and emission spectra

Customers also purchased with this product
Cyanine5.5 carboxylic acid
Cyanine5.5 dye, free acid form, unactivated. The dye can be considered non-reactive for most applications.AF 488 carboxylic acid
AF 488 carboxyl derivative can be used as a reference standard or for synthesizing various AF488 esters.ROX amine, 6-isomer
An amine derivative of ROX (Rhodamine X, Rhodamine 101) dye that is reactive toward electrophiles. It can also be used for enzymatic transamination labeling. Pure 6-isomer.General properties
| Appearance: | dark red crystals |
| Molecular weight: | 654.84 |
| CAS number: | 1121756-11-3 (inner salt); 1941997-61-0 (sodium salt) |
| Molecular formula: | C30H35N2KO8S2 |
| IUPAC name: | 3H-Indolium, 2-[3-[1-(5-carboxypentyl)-1,3-dihydro-3,3-dimethyl-5-sulfo-2H-indol-2-ylidene]-1-propen-1-yl]-1,3,3-trimethyl-5-sulfo-, inner salt, potassium salt |
| Solubility: | Well soluble in water, DMF, DMSO (0.55 M = 360 g/L). Practically insoluble in non-polar organic solvents. |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 548 |
| ε, L⋅mol−1⋅cm−1: | 162000 |
| Emission maximum, nm: | 563 |
| Fluorescence quantum yield: | 0.1 |
| CF260: | 0.03 |
| CF280: | 0.06 |
Product citations
- Vallmitjana, A.; Torrado, B.; Dvornikov, A.; Ranjit, S.; Gratton, E. Blind Resolution of Lifetime Components in Individual Pixels of Fluorescence Lifetime Images Using the Phasor Approach. Journal of Physical Chemistry B, 2020, 124(45), 10126–10137. doi: 10.1021/acs.jpcb.0c06946
- Wu, Q.; Higler, R.; Kodger, T.E.; van der Gucht, J. Particle Dynamics in Colloid–Polymer Mixtures with Different Polymer Architectures. ACS Applied Materials & Interfaces, 2020, 12(37), 42041-42047. doi: 10.1021/acsami.0c07153
- Cho, U.; Riordan, D.P.; Ciepla, P.; Kocherlakota, K.S.; Chen, J.K.; Harbury, P.B. Ultrasensitive optical imaging with lanthanide lumiphores. Nature Chemical Biology, 2018, 14(1), 15–21. doi: 10.1038/nchembio.2513
- Spears, B.R.; Marin, M.A.; Chaker, A.N.; Lampley, M.W.; Harth, E. Precise Microscale Polymeric Networks through Piezoelectronic Inkjet Printing. ACS Biomaterials Science & Engineering, 2016, 2(8), 1265–1272. doi: 10.1021/acsbiomaterials.6b00175
Cy® is a trademark of GE Healthcare.
Short link - lumiprobe.com/sh/p/1C
The count of items is incorrect.











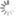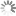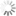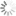
 $
$ 
