TAMRA phosphoramidite, 5-isomer
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 2497-250mg | 250 mg | please inquire | in stock (2 pcs) | |
| 2497-1g | 1 g | please inquire | in stock (2 pcs) | |
| 2497-5g | 5 g | please inquire | in stock (1 pcs) |

This phosphoramidite is used for synthesis of oligonucleotides 5’-labeled with TAMRA.
TAMRA (carboxytetramethylrhodamine) is a xanthene dye from the rhodamine family with emission in the orange spectrum range (maximum at 563 nm). This fluorophore is traditionally used as a FRET-acceptor (and a quencher) in a pair with fluorescein (FAM) due to significant overlapping of their spectra. Thus, this phosphoramidite is convenient for the synthesis of dual-labeled probes TaqMan, which contain 5’-terminal TAMRA and FAM in the middle of the sequence or at the 3’-end (using Fluorescein dT Phosphoramidite and FAM CPG, respectively).
TAMRA 5’-labeled oligonucleotides are commonly used for quantitative PCR and fragment analysis (for example, for microsatellite marker analysis) because the equipment available has a detection channel for TAMRA frequently.
The TAMRA dye is not stable in the presence of ammonium and sterically non-hindered primary amines, so it is strongly recommended to follow specified conditions for labeled oligonucleotide deprotection.
Usage
Coupling: 7.5 min.
Deprotection: tret-buthylamine : methanol : water 1 : 1 : 3 (v/v/v) («TAMRA cocktail») for 6 hours at 60 °C, then cool down to room temperature.
Due to complete and irreversible degradation of the TAMRA dye, do NOT use aqueous ammonium and AMA for deprotecting a modified oligonucleotide from the solid-phase support.
Absorption and emission spectra of 5-TAMRA

Customers also purchased with this product
HEX azide, 6-isomer
Azide of green-yellow emitting HEX dye for conjugation with oligonucleotides via [3+2]-dipolar cycloaddition.ROX azide, 5-isomer
Azide derivative of ROX (Rhodamine X, Rhodamine 101) dye for biomolecule labeling via click chemistry. Pure 5-isomer.get free express delivery
DusQ® 21 CPG 500
DusQ® 21 is a quencher for dyes in red part of the spectrum, including Cyanine5 and Cyanine5.5.get free express delivery
General properties
| Appearance: | purple solid |
| Mass spec M+ increment: | 589.60 |
| Molecular weight: | 727.83 |
| Molecular formula: | C40H50N5O6P |
| Solubility: | good in acetonitrile, dichloromethane |
| Quality control: | NMR 1H, HPLC-MS (95%), coupling test |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 541 |
| ε, L⋅mol−1⋅cm−1: | 84000 |
| Emission maximum, nm: | 567 |
| CF260: | 0.32 |
| CF280: | 0.19 |
Oligo synthesis details
| Diluent: | acetonitrile |











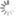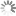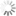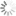
 $
$ 
