FAM phosphoramidite, 5-isomer
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
|
2639-500mg
/ Easily soluble lyophilised form in 8 mL septum
|
500 mg |
–
|
in stock | |
|
2639-1g
/ Easily soluble lyophilised form in 20 mL septum
|
1 g |
$190
|
in stock | |
|
2639-5g
/ in 30 mL septum
|
5 g |
$740
|
20 days | |
|
2639-10g
/ in 30 mL septum
|
10 g |
$1290
|
20 days | |
| 2639-50g | 50 g |
please inquire
|
20 days | |
| 2639-100g | 100 g |
please inquire
|
20 days |

Standard fluorescein (FAM) phosphoramidite for 5'-terminal oligonucleotide labeling, high isomeric purity single isomer. This highly purified amidite reagent ensures excellent coupling results with various synthesizers.
Samples and special offers are available for oligo manufacturers. Contact us for bulk supplies of this reagent.
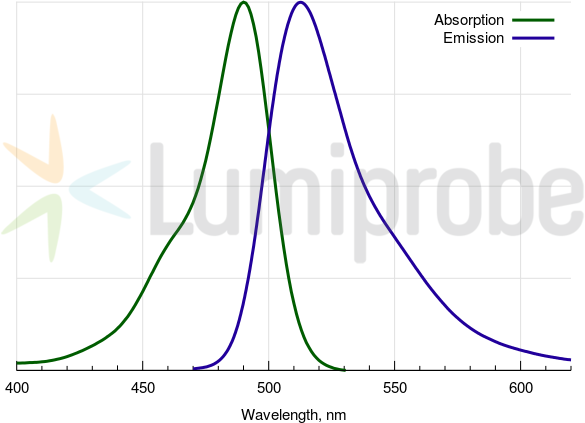
FAM absorbance and emission spectra
Customers also purchased with this product
General properties
| Appearance: | off white solid |
| Molecular weight: | 843.94 |
| CAS number: | 147566-42-5 |
| Molecular formula: | C46H58N3O10P |
| Solubility: | Good solubility in acetonitrile and DCM |
| Quality control: | NMR 1H and 31P, HPLC-MS (95+%), isomeric purity > 97% |
| Storage conditions: | Storage: 12 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 492 |
| ε, L⋅mol−1⋅cm−1: | 72000 |
| Emission maximum, nm: | 517 |
| Fluorescence quantum yield: | 0.93 |
| CF260: | 0.22 |
| CF280: | 0.17 |
Oligo synthesis details
| Diluent: | acetonitrile |
| Coupling conditions: | coupling time 10 min |
| Cleavage conditions: | ammonia, 2 h at room temperature |
| Deprotection conditions: | identical to protected nucleobases; when AMA is used, deblock with ammonia alone for 30 min, then add methylamine |
Product citations
- Zharkov, T. D.; Markov, O. V.; Zhukov, S. A.; Khodyreva, S. N.; Kupryushkin, M. S. Influence of Combinations of Lipophilic and Phosphate Backbone Modifications on Cellular Uptake of Modified Oligonucleotides. Molecules, 2024, 29(2), 452. doi: 10.3390/molecules29020452
- Bauer, I.; Ilina, E.; Zharkov, T.; Grigorieva, E.; Chinak, O.; Kupryushkin, M.; Golyshev, V.; Mitin, D.; Chubarov, A.; Khodyreva, S.; Dmitrienko, E. Self-Penetrating Oligonucleotide Derivatives: Features of Self-Assembly and Interactions with Serum and Intracellular Proteins. Pharmaceutics, 2023, 15(12), 2779. doi: 10.3390/pharmaceutics15122779
- Petrunina, N.A.; Lebedev, V.V.; Kirillova, Y.G.; Aralov, A..V.; Varizhuk, A.M.; Sardushkin, M.V. DNA Intercalated Motifs with Non-Nucleoside Inserts. Russian Journal of Bioorganic Chemistry, 2021, 47(6), 1341–1344. doi: 10.1134/S1068162021060212
This Product is offered and sold for research purposes only. It has not been tested for safety and efficacy in food, drug, medical device, cosmetic, commercial or any other use. Supply does not express or imply authorization to use for any other purpose, including, without limitation, in vitro diagnostic purposes, in the manufacture of food or pharmaceutical products, in medical devices or in cosmetic products.
Short link - lumiprobe.com/sh/p/7z
The count of items is incorrect.

























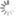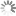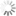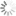
 $
$ 
