Cyanine3 carboxylic acid
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| 11090 | 1 mg |
$110
|
in stock | |
| 21090 | 5 mg |
$210
|
in stock | |
| 41090 | 25 mg |
$410
|
in stock | |
| 51090 | 50 mg |
$695
|
in stock | |
| 61090 | 100 mg |
$1190
|
in stock |

Free Cyanine3 carboxylic acid (Cy3® carboxylic acid analog), non-activated dye. Non-sulfonated reagent, with good solubility in organic solvents and limited aqueous solubility. The dye can be used as a non-reactive fluorophore for control experiments, and for calibration.
For coupling with amines, and labeling, consider using Cyanine3 NHS ester, or water-soluble sulfo-Cyanine3 NHS ester.
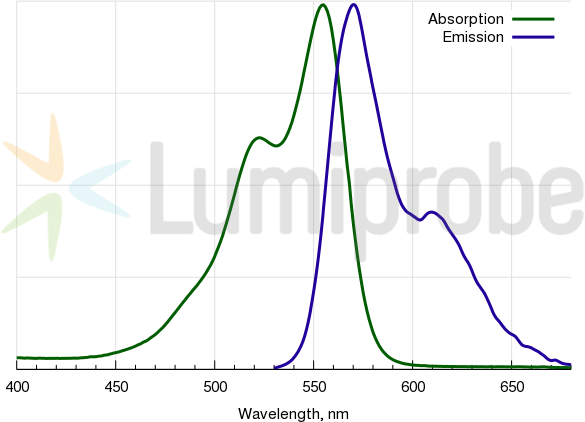
Cyanine3 absorbance and emission spectra
Customers also purchased with this product
General properties
| Appearance: | red powder |
| Molecular weight: | 493.08 |
| CAS number: | 1361402-15-4 (inner salt), 1032678-01-5 (chloride), 1251915-29-3 (iodide) |
| Molecular formula: | C30H37ClN2O2 |
| Solubility: | soluble in organic solvents (DMF, DMSO, dichloromethane), poorly soluble in water (1.8 g/L = 4.0 mM) |
| Quality control: | NMR 1H, HPLC-MS (95%) |
| Storage conditions: | Storage: 24 months after receival at -20°C in the dark. Transportation: at room temperature for up to 3 weeks. Avoid prolonged exposure to light. Desiccate. |
| MSDS: | Download |
| Product specifications |
Spectral properties
| Excitation/absorption maximum, nm: | 555 |
| ε, L⋅mol−1⋅cm−1: | 150000 |
| Emission maximum, nm: | 570 |
| Fluorescence quantum yield: | 0.31 |
| CF260: | 0.04 |
| CF280: | 0.09 |
Product citations
- Herkert, E.K.; Lau, L.; Pons Lanau, R.; Garcia-Parajo, M.F. Hexagonal Plasmonic Arrays for High-Throughput Multicolor Single-Molecule Studies. ACS Applied Materials & Interfaces, 2024, 16(31), 41271-41280. doi: 10.1021/acsami.4c04744
- De Pauw, E.; Chen, Y.; De Keersmaecker, H.; De Coninck, E.; De Smet, L.; De Geest, B.; Braeckmans, K.; Vervaet, C.; Vanhoorne, V. Drying Behaviour and Visualization of Surfactants after Co-Spray Drying of Surfactant-Stabilized Aqueous Suspensions. International Journal of Pharmaceutics, 2023, 643, 123231. doi: 10.1016/j.ijpharm.2023.123231
- Xu, S.; Zhang, P.; Heing-Becker, I.; Zhang, J.; Tang, P.; Bej, R.; Bhatia, S.; Zhong, Y.; Haag, R. Dual Tumor- and Subcellular-Targeted Photodynamic Therapy Using Glucose-Functionalized MoS2 Nanoflakes for Multidrug-Resistant Tumor Ablation. Biomaterials, 2022, 290, 121844. doi: 10.1016/j.biomaterials.2022.121844
- Mattila, J. T.; Beaino, W.; White, A. G.; Nyiranshuti, L.; Maiello, P.; Tomko, J.; Frye, L. J.; Fillmore, D.; Scanga, C. A.; Lin, P. L.; Flynn, J. L.; Anderson, C. J. Retention of 64Cu-FLFLF, a Formyl Peptide Receptor 1-Specific PET Probe, Correlates with Macrophage and Neutrophil Abundance in Lung Granulomas from Cynomolgus Macaques. ACS Infect. Dis., 2021, 7(8), 2264–2276. doi: 10.1021/acsinfecdis.0c00826
Cy® is a trademark of GE Healthcare.
Short link - lumiprobe.com/sh/p/W
The count of items is incorrect.
























 $
$ 
